Fibroblast growth factor-23 (FGF23) is a bone-derived hormonal regulator of mineral metabolism with emerging functions in the bone marrow. FGF23 has been reported to negatively regulate erythropoiesis [PMID:24509850] and promote hematopoietic progenitor mobilization from the bone marrow [PMID:33512467]. Elevated FGF23 levels in the circulation have been described in iron-deficient subjects and also in subjects with the iron-loading anemia β-thalassemia. Recently, we reported bone marrow sinusoidal endothelial cells (BM-SEC) as a novel site of Fgf23 upregulation in mice with chronic iron-deficiency anemia and acute, phlebotomy-induced anemia [PMID:37417950].
We and others have detected Fgf23 mRNA elevation in total bone marrow of Hbb th3/+ mice, a model of non-transfusion dependent β-thalassemia that exhibits systemic iron loading. In the present study, we sought to clarify sites of Fgf23 promoter activity in Hbb th3/+ bone marrow using a reporter allele in which the enhanced green fluorescent protein (eGFP) coding sequence is knocked into the endogenous Fgf23 locus ( Fgf23 eGFP). We generated Hbb +/+ and Hbb th3/+ female littermates that carried either one ( Fgf23 +/eGFP) or zero ( Fgf23 +/+) copies of the Fgf23 reporter allele, which were raised on a standard rodent diet (200 mg/kg iron, 1.0% calcium, 0.7% phosphorus). Fgf23 eGFP/eGFP homozygotes were not generated due to known early growth retardation and mortality in Fgf23 null mice. After blood and tissue collection at 8 weeks of age, phenotypes of the 4 Hbb-Fgf23 genotype combinations were compared by two-way ANOVA.
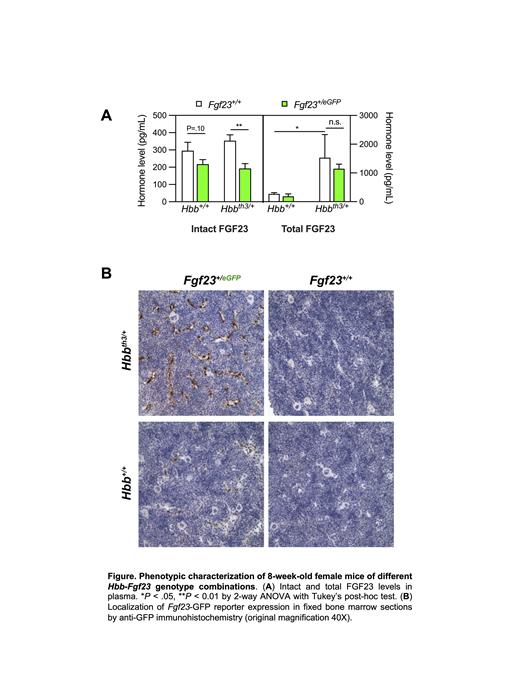
Compared to mice with intact Hbb and Fgf23 alleles ( Hbb +/+ Fgf23 +/+), Hbb th3/+ Fgf23 +/+ mice showed markedly elevated plasma levels of “total” FGF23 (sum of intact, active hormone and C-terminal cleaved fragments). Plasma total FGF23 levels in Hbb th3/+ Fgf23 +/+ and Hbb th3/+ Fgf23 +/eGFP mice were similar, indicating utility of the latter genotype for interrogating sites of increased Fgf23 expression in murine b-thalassemia. We therefore used anti-GFP immunohistochemistry in formalin-fixed bone marrow sections to assess Fgf23 eGFP reporter allele expression in the context of tissue architecture. Hbb th3/+ Fgf23 +/eGFP mice showed GFP expression in BM-SEC throughout the bone marrow, which was more intense than in non-anemic Hbb +/+ Fgf23 +/eGFP mice; controls lacking the Fgf23 eGFP allele confirmed anti-GFP antibody specificity. Hbb th3/+ mice also showed Fgf23 eGFP reporter expression in the thymic vasculature but not vasculature of the liver, spleen, or kidney.
In contrast to their marked elevation in plasma total FGF23, Hbb th3/+ Fgf23 +/+ mice showed plasma levels of the intact, biologically-active FGF23 hormone that overlapped with levels in Hbb +/+ Fgf23 +/+ controls. Interestingly, compared with Hbb th3/+ Fgf23 +/+ mice, Hbb th3/+ Fgf23 +/eGFP mice showed 45% lower plasma intact FGF23, suggesting an effect of Fgf23 gene dosage. Given prior reported effects of FGF23 on hematopoiesis, we assessed if Fgf23 gene dosage modulated the severity of ineffective erythropoiesis in Hbb th3/+ mice. Compared to Hbb +/+ Fgf23 +/+ controls, Hbb th3/+ Fgf23 +/+ mice showed reductions in RBC, hemoglobin, and mean corpuscular hemoglobin, as well as increases in RDW and in spleen weight (reflecting extramedullary hematopoiesis). However, the severity of these parameters was not modified by heterozygous Fgf23 disruption ( Hbb th3/+ Fgf23 +/eGFP).While FGF23 has been reported to induce left ventricular hypertrophy [PMID:21985788], Hbb th3/+ Fgf23 +/+ and Hbb th3/+ Fgf23 +/eGFP mice showed similarly increased heart-to-body weight ratios compared to Hbb +/+ Fgf23 +/+ controls. In non-thalassemic mice, heterozygous Fgf23 disruption ( Hbb +/+ Fgf23 +/eGFP) caused a trend ( P=0.10) towards lower plasma intact FGF23 while not impacting hematological parameters or spleen weight.
In summary, by showing that Fgf23 gene dosage modulates plasma intact FGF23 levels in Hbb th3/+ mice without altering severity of anemia or splenomegaly, our data suggest that moderate reductions in circulating levels of the intact FGF23 hormone do not alter the severity of ineffective erythropoiesis in Hbb th3/+mice at 8 weeks of age. Our finding that β-thalassemic mice exhibit Fgf23 upregulation in BM-SEC lays the groundwork for future investigation of the functional effects of locally-produced FGF23 in the thalassemic hematopoietic stem cell niche.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal